Detection of exogenous sugars in pineapple juice using compound-specific stable hydrogen isotope analysis
Simon D. Kelly, Aiman Abrahim, Peter Rinke and Andrew Cannavan
Food and Environmental Protection Laboratory, Joint FAO/IAEA Division of Nuclear Applications in Food and Agriculture, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, Vienna International Centre, PO Box 100, 1400 Vienna, Austria
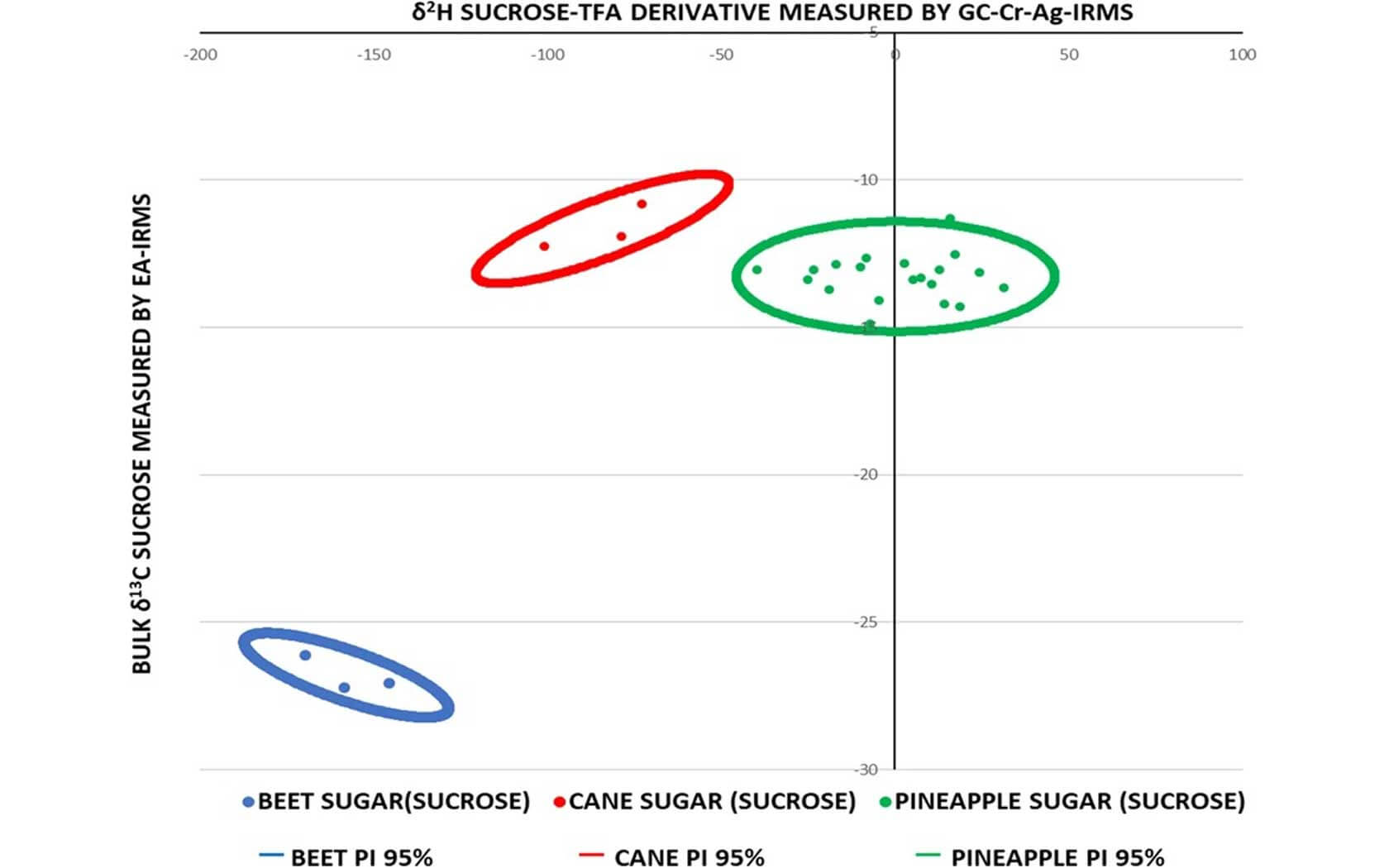
Isotope analysis is the preferred technique used to identify the addition of low-cost sugars to fruit juices and are difficult to subvert because of the high cost of changing the isotopic ratios of the sugars. The global δ13C values and SNIF-NMR (2H/1H) values of pineapple, cane and maize sugars overlap to such an extent that they cannot reliably detect their addition in isolation or when masked by other adulterations. To overcome this issue, a new procedure has been developed by Abrahim, Cannavan and Kelly with the use of a volatile fluorinated derivative of carbohydrate facilitating the rapid δ2H measurement of carbon bound non-exchangeable hydrogen by compound-specific stable isotope analysis. This permits the global hydrogen isotopic composition of individual mono- and disaccharides to be reliably measured with a precision better than 3 ‰. The procedure utilizes a simple one-step reaction to substitute the exchangeable hydroxyl-hydrogens on sugars from pineapple juice with trifluoroacetate (TFA) derivatives that are sufficiently volatile to be separated by gas chromatography and measured using our BiovisION system equipped with a GC5 inlet (GC-GC5-IRMS).
Twenty authentic commercial production samples of single-strength pineapple juice, pineapple puree and pineapple juice concentrate were obtained from SGF International e.V. The major sugars in pineapple juice (sucrose, glucose and fructose) were separated as their TFA derivatives by gas chromatography and then passed into a micro-bore furnace containing chromium metal particles and silver wool maintained at 1200 °C. Under typical high temperature conversion conditions around 1400 °C in an open ceramic tube coated with carbon, the formation of HF effects the repeatability of δ2H measurements. This issue was avoided by utilizing Elementar’s ChromeHD technology where organic compounds undergo on-line reduction over chromium metal (Cr/HTC) to H2 at 1200 °C with the GC5 in accordance with the method described on their previous publication. The carbon isotope measurements to confirm the juices’ C3 and C4 identities were performed by combustion of the bulk sugar obtained after the fruit juice acid separation using our BiovisION system and then subsequently normalized to the V-PDB scale using certified reference materials USGS40 and USGS41 (L-glutamic acids).
To demonstrate the relationship between pineapple sucrose carbon-bound non-exchangeable (CBNE) δ2H values and those obtained with added cane sugar, the data were compared with freshly squeezed pineapple juice sucrose CBNE δ2H values +14.17 ± 0.69 ‰. This worked as a simulation of juice adulteration with cane sugar sucrose, with a CBNE δ2H value of −101.12 ± 2.8 ‰. The overall measurement precision for the 20 authentic pineapple juices reported in this study with the BiovisION system ranged from 0.2 to 2.2 ‰ with an average value of 1.1 ‰. The bulk δ13C measurement of the sugars isolated from the 20 authentic pineapple juices had an outstanding precision as well. It was measured in triplicate by EA-IRMS and the precision ranged from 0.02 to 0.40 ‰ with an average value of 0.12 ‰.
Please find the full paper here: https://doi.org/10.1038/s41538-021-00092-5